Paradigm Shift in R&D
Coopetition is the KeyBringing new products to market has been costly, with low R&D efficiencies at the back of high risk of failure, pricing pressures, and a lengthy R&D path. Companies are finding new avenues to discover and conduct research to maximize ROI on their strategic assets with the help of digitalization, open innovation, and shared models. Companies are focusing on projects that could be eligible for a shorter R&D cycle and qualifies for quick regulatory approvals. The innovators are opening up for partnerships with companies that are into similar or other business segments, including close competitors.
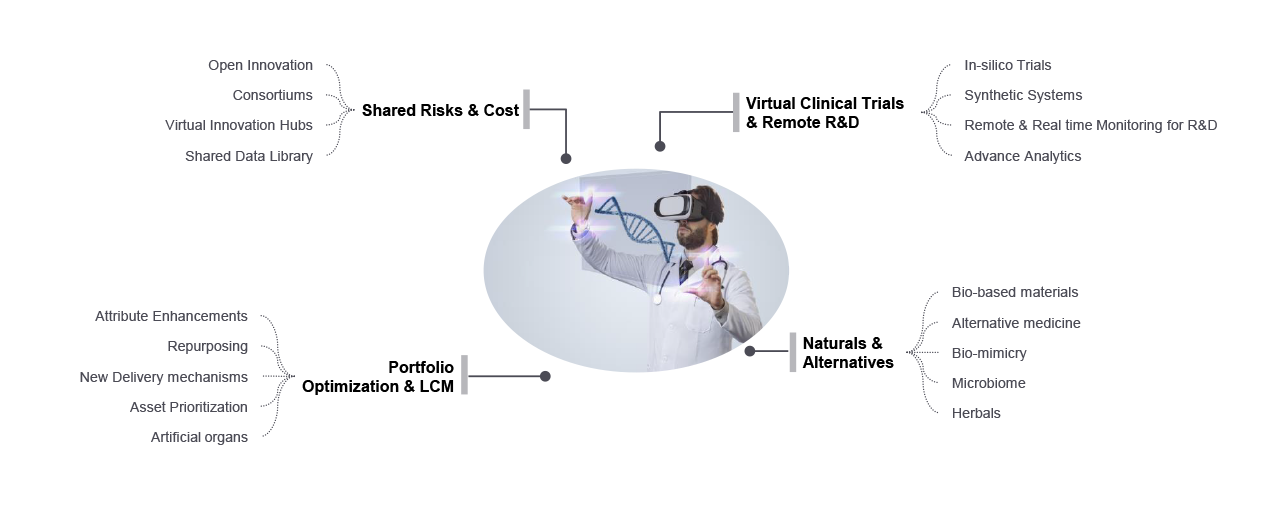
Key Trends
Open Innovation
- External Innovation funds
- Non-allied collaborations
- Partner enabled innovation
- Shared resource pool
Virtual Clinical Trials
- Hybrid trial models
- Decentralized trials
- E-technology (recruiting, consent)
- Remote patient recruitment
Repurposing
- New regulatory pathways
- Rare disease &
- Indication extensions
- Rx to OTC switch
Bio-based Materials
- Plant based
- Natural pure form
- Sustainable sourcing
Shared Data Library
- Open data platforms
- Secondary use of data
- Digitization
- Cel line library
Advanced Analytics
- Clinical algorithms
- Data-driven analytics and outcomes
- Predictive analytics
- PAT
New Delivery Mechanisms
- Formulations and formats
- Drug-Device combinations
- Connected delivery devices
Microbiome
- Probiotics
- Combination of pre & probiotics, herbs
- Skin & gut health
Perspectives

Life SciencesParadigm Shift in R&D
Partner Enabled Innovation Models

Life SciencesParadigm Shift in R&D
Evolution of Process Analytical Technologies
Rare Cancers: Challenges and Roadmap for R&D Innovations
Life SciencesParadigm Shift in R&D

Challenges and Drivers of Translational Research
Life Sciences

Virtual Clinical Trials
Life SciencesParadigm Shift in R&D

Blockchain in Electronic Health Records (EHR) – A Distant Future but a Step in a Right Direction
Life SciencesEvolving Ecosystems
Business Objectives
Some examples of diverse business objectives we have worked with our clients
Strategic
- Which innovation trends should be capitalized on to introduce new R&D-based offerings?
- What structural changes are required to implement patient-centric clinical trials?
- How companies have overcome the implementation-related challenges in virtual clinical trials?
Tactical
- What are shared funding models to reconfigure high R&D costs & related risks?
- Which technologies can be used to improve the productivity of virtual clinical trials?
- Which new promising frontiers can help mitigate the high risk of R&D failures?
Operational
- How companies are increasing therapy adherence in clinical trials using digital solutions?
- Which are the attractive chronic therapy segments from application perspectives?





