Transforming Health of Companion Animals through Point-of-Care Diagnosis
Introduction
Companion animals majorly consist of dogs and cats that are integrated into the family and considered as part of the family. These companion animals can act as carriers of diseases that infect humans. Diseases transmitted between animals and humans are known as zoonoses, which are mainly spread through vectors, such as flies, ticks, and mosquitoes. Therefore, the care and concern by pet owners regarding the health and wellbeing of companion animals are increasing. Dogs and cats are susceptible to human tuberculosis caused by Mycobacterium tuberculosis, methicillin-resistant Staphylococcus aureus, and influenza A. Routine testing of companion animals during clinical visits is critical to control and prevent the spread of such diseases.
Early, accurate, and real-time diagnosis is critical for better health
Early diagnosis plays a vital role in making critical clinical decisions. In traditional diagnostic systems, samples are collected from clinics and reach the central laboratory for evaluation. Thereafter, test results are communicated to the veterinarian. The process delays the time for treatment from the identification of symptoms by the veterinarian to the provision of appropriate therapy. Thus, Point-Of-Care (POC) devices are expected to improve this situation by providing accurate and reliable results in real-time.
Overview of Point-of-Care Devices
Point-of-care testing devices have been available in the veterinary practice for a long time. The technology evolved from the diagnosis of a simple pathogen to performing PCR-based techniques. Advances in the lateral flow technology, such as conjugation methods, helped increase the sensitivity of point-of-care devices. Kits based on the rapid immunochromatographic techniques are being widely accepted, as they are easy-to-use, cost-effective, and offer reproducible results. 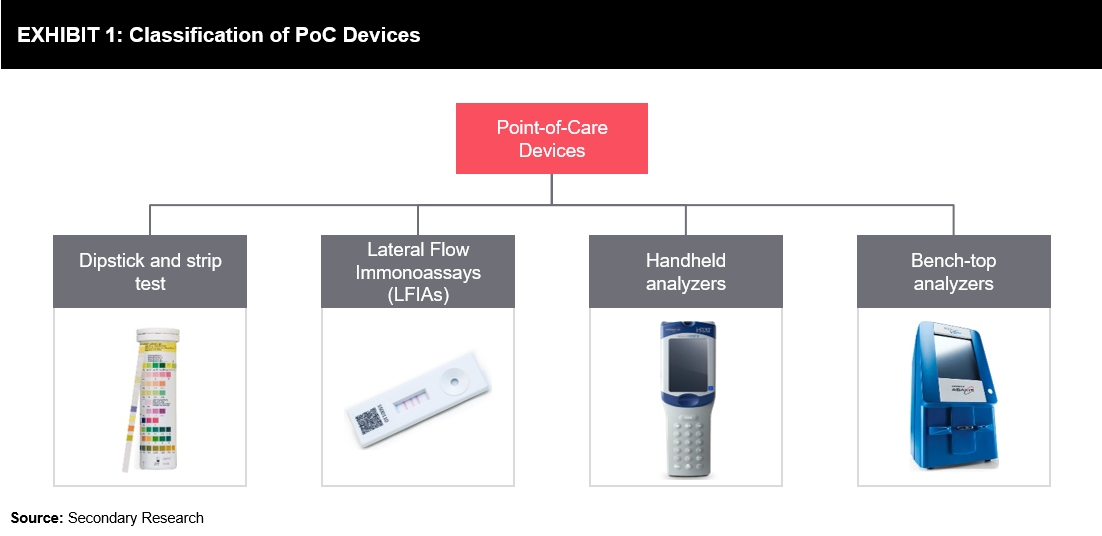
Early-stage portable testing devices are available for measuring blood cell differentials in animals. The automated cell counter developed for humans is also altered for animal use. Further, technological developments have led to more advanced small bench-top versions of testing devices that are commercially available. These devices are widely used in practice and can be found even in small veterinary clinics, serving the purpose of instant diagnosis. Portable testing devices can assess the degree of infection accurately by examining abnormalities in white blood cell counts.
Rapid analysis of blood gases and electrolyte detection technologies are adopted for various veterinary applications. Apart from clinics and portable veterinary facilities, these analyzers are also employed at equine competitions to analyze the electrolyte balance. Maintenance of electrolyte balance is challenging during such strenuous competitions that animals are exposed to.
The lateral flow immunoassay test for heartworm, Lyme disease, canine parvovirus, Giardia, Ehrlichia, Anaplasma, and feline leukemia/immunodeficiency virus is currently available. This test provides visually detectable results in 10-15 minutes. Such cost-efficient testing platforms offer multiplexing capabilities, which further reduces the cost and time of diagnosis.
Heartworm disease is a serious and fatal disease most commonly affecting dogs. According to the American Heartworm Society (AHS) incidence survey, the average number of heartworm cases in dogs in 2016 was 21.6%, higher than the number of cases diagnosed in 2013. Feline Leukemia Virus (FeLV) and Feline Immunodeficiency Virus (FIV) are some of the most common diseases in cats; PoC tests are helping veterinarians in undertaking treatment decisions. Exhibit 2 offers an illustrative representation of the incidence of heartworm disease in the US in 2016.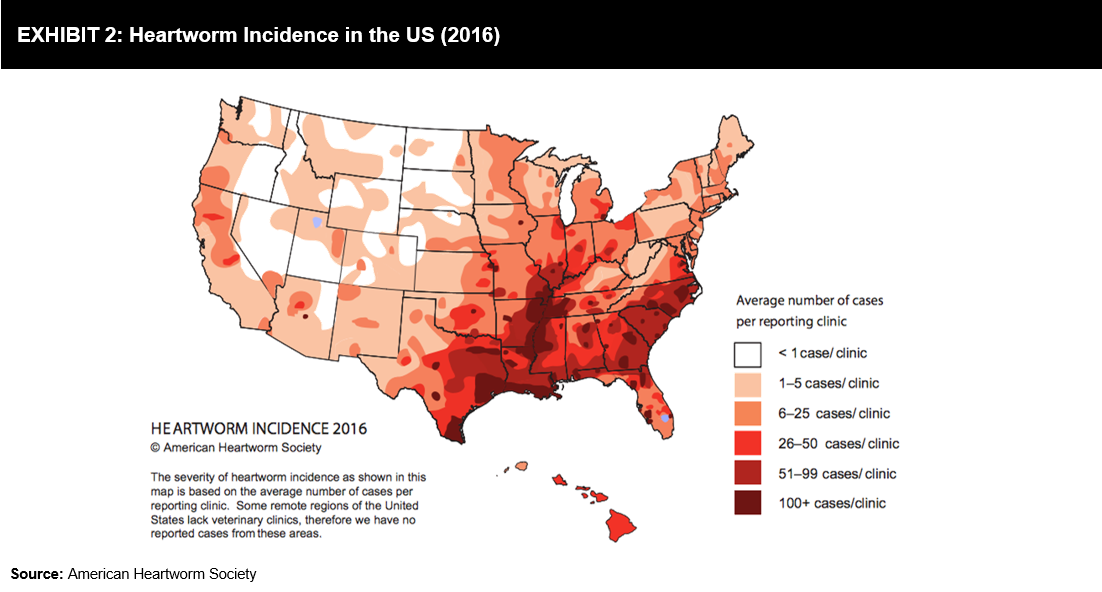
Recent reports indicate the increasing prevalence of diabetes in companion animals. It has been identified (refer to Exhibit 3) that 1 in 230 cats and 1 in 308 female dogs are at an increased risk of getting diagnosed with diabetes. A four-year study (2006-2010) conducted by the Banfield Pet Hospital in the US stated that diabetes diagnosis in pets increased by 32% in dogs and 16% in cats.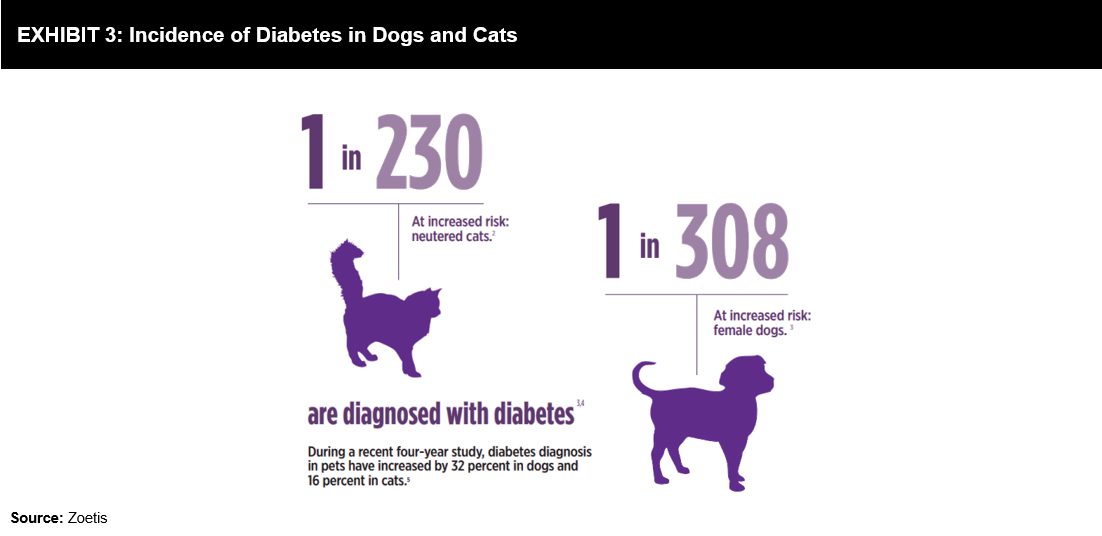
Vcheck V200, a fully quantitative PoC analyzer, provides an accurate diagnosis for dogs and cats. Using this analyzer, pancreatitis markers (cPL, fPL), inflammatory markers (cCRP, fSAA), and T4 measurement can be done in real-time.
Another class of devices that are useful for veterinarians in quick quantitative analysis is handheld devices. These devices are preferred for measuring blood gas, electrolytes, clinical chemistry, lactate concentration, and hematology. Such easy-to-use devices are ideal for critical care departments, operating/monitoring rooms, and small clinics, where quick diagnosis results are essential for monitoring and treatment. The complete test results are available in 2-3 minutes.
Lateral flow kits and handheld devices are able to provide quick solutions; however, for accurate diagnoses of a few diseases, such as canine distemper virus and canine parvovirus, detection methods require Polymerase Chain Reaction (PCR). Real-time PCR devices play an important role in the detection of infectious diseases. With the availability of mini/portable PCR devices, such as pocket PCR and kits, PCR-based tests can be performed in clinics, academic medical centers, and research institutes.
Snap ELISA test (refer to Exhibit 4) developed on the principle of ELISA, proves to be a reliable, sensitive, and specific diagnostic test. Snap ELISA uses reverse directional flow to amplify the signal by enzymatic reaction step, eliminate nonspecific binding, and display visible results due to a distinct colored reaction. This test offers more sensitivity and specificity as compared to lateral flow systems, which are based on colloidal gold or colored latex particles attached to diagnostic reagents. Test kits are available for Lyme disease, Ehrlichia, heartworm, Leishmaniasis, Anaplasma, feline leukemia virus, feline immunodeficiency virus, parvovirus, Giardia, feline heart disease, and canine or feline pancreatitis. The assay can be completed in 6-10 minutes, depending on the test.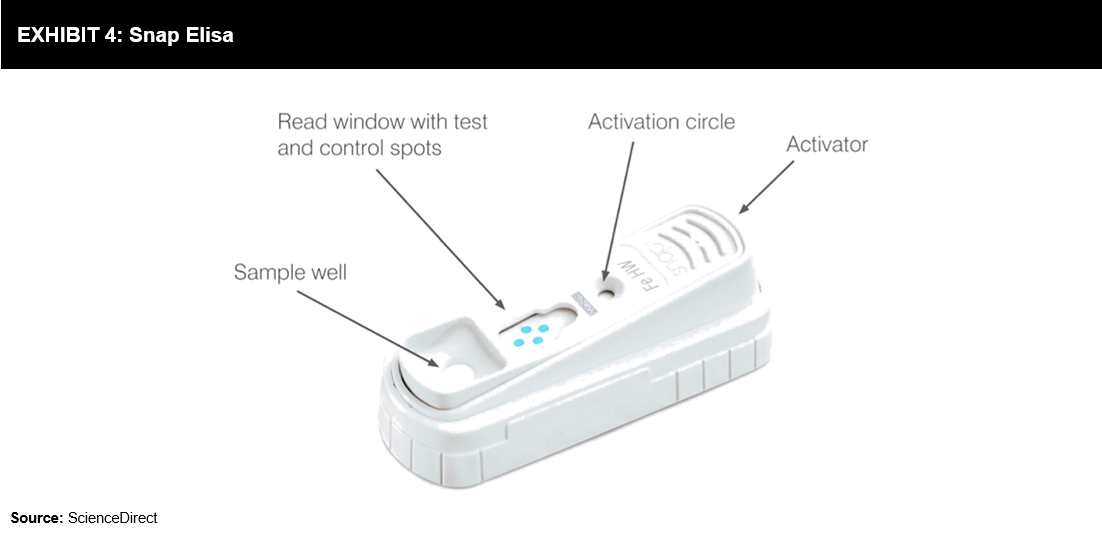
Based on recent estimates offered by the American Pet Products Association’s 2017-2018 National Pet Owners Survey, 60.2 million households in the US hold 89.7 million dogs and 47.1 million households hold 94.2 million cats; the United Kingdom has 9 million dogs and 7.5 million cats. The preventive care screening performed at more than 5000 practices indicated results that warranted further action (refer to Exhibit 5). These growing numbers offer opportunities for the growth of PoC devices. In 2017, companion animals accounted for 69% of the total rapid diagnostics market.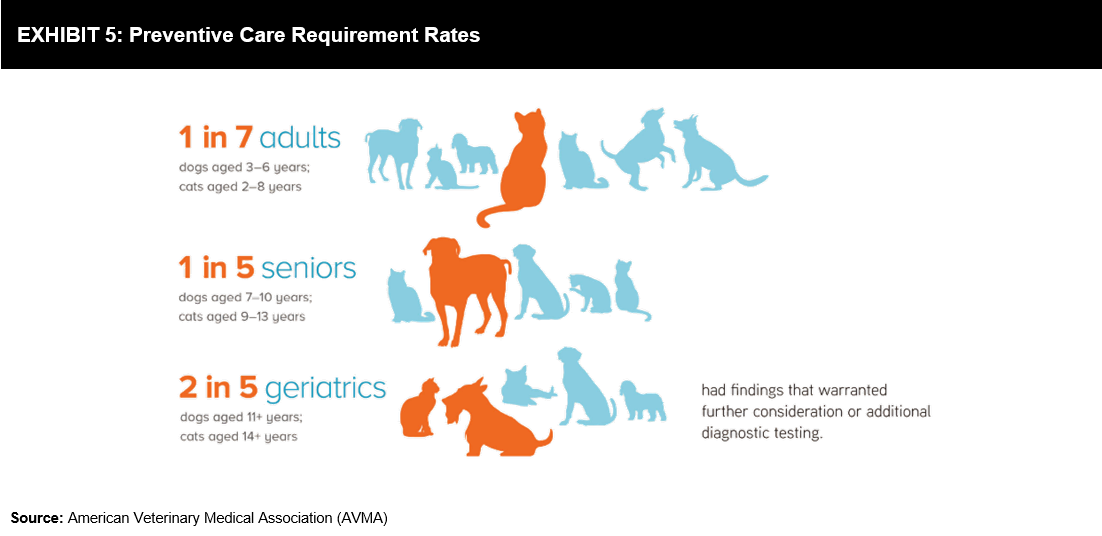
Guidelines for Veterinary POCT
With the increasing acceptance of Point-of-Care testing (POCT), the American Society for Veterinary Clinical Pathology (ASVCP) has developed Quality Assurance (QA) guidelines veterinary POCT. Major recommendations are listed below:
- Taking a formalized approach to POCT within the facility
- Use of written policies, standard operating procedures, forms, and logs
- Operator training, including a periodic assessment of skills
- Assessment of instrument analytical performance and use of both, statistical quality control and external quality assessment programs
- Use of properly established or validated reference intervals
- Accurate reporting of patient results
The guidelines also suggest the use of a validated 13s control rule for interpretation of control data for analytical instruments. The implementation of these guidelines by veterinarians and veterinary technicians shall improve the management of POCT in their clinical or research settings as well as address quality issues of small chemistry and hematology instruments.
Challenges and Opportunities
The existing PoC devices can detect a wide spectrum of diseases in the veterinary clinic. However, there still exists the need to accurately detect a few infectious diseases in animals. Understanding the importance of such devices in maintaining animal health, the European Union (EU) has funded the project named POC4PETS for the development of point-of-care diagnostics for rapid and cheap pathogen detection of companion animals. The project aims at the development of new diagnostic tests for Leishmania detection, feline calicivirus, and feline herpesvirus.
Diagnostic tests currently available are able to meet a majority of the requirements in veterinary practice; however, these tests come with certain limitations. Emerging technologies need to offer low-cost, rapid, and reliable results. Microfluidics has created new opportunities in human diagnostics; this technology has immense potential to address current challenges in the field of veterinary diagnostics.
Further, research activities are underway in the area of detecting different nucleic acid detection technologies, on-site PCR, mini-array probing, isothermal amplification, and economical solutions for measuring DNA/RNA for diagnosis of infections. Technological advancements will widen and improve the range of POC tests available.
Veterinarians are comfortable transitioning from traditional systems to PoC devices, provided the tests to deliver reliable results needed for effective clinical decisions. Though the cost of these technologies is reducing gradually, storage stability and ease-of-use are critical for their overall acceptance. The availability of pet insurance for pet owners has helped animal diagnostics become a lucrative market. However, increased pet care costs might act as a hindrance to market growth.
Conclusion
Point-of-care devices for companion diagnostics include dipstick and strip test, LFIAs, and handheld & bench-top analyzers. These diagnostic solutions are helping veterinarians ensure a quick diagnosis of various diseases, including diabetes, heartworm, Lyme disease, canine parvovirus, FeLV, FIV, etc. Accurate and real-time diagnosis is critical for better health. With the increase in the prevalence of zoonotic diseases and other infectious diseases, there exists a need for new technologies to provide low-cost rapid diagnostic solutions. R&D investments are essential to provide viable solutions for challenging future needs. The increasing demand for fast testing and portable devices for point-of-care services is anticipated to provide market players with prospective growth opportunities in the near future.




































