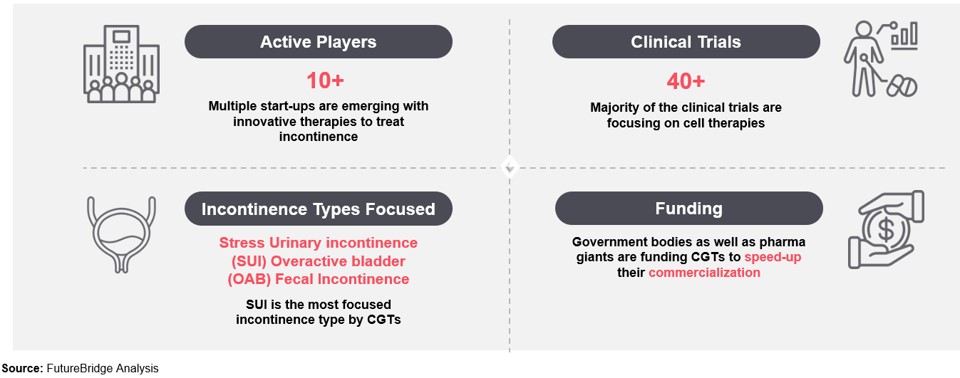
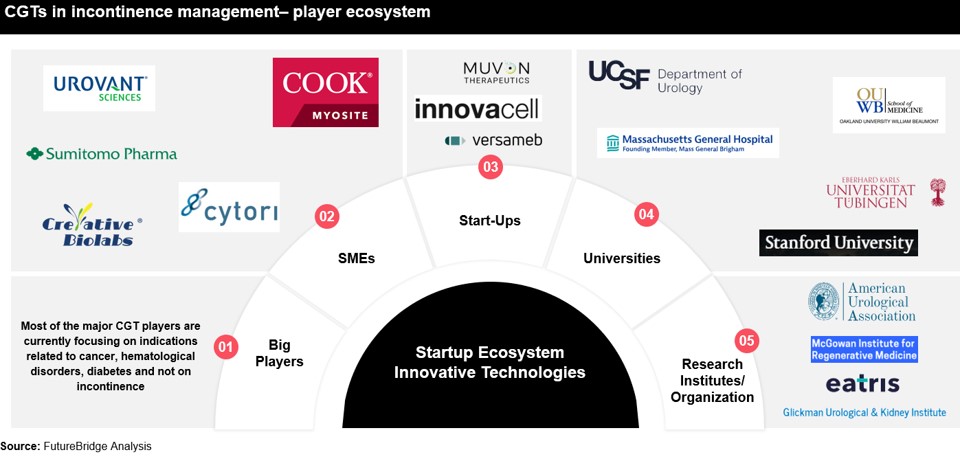
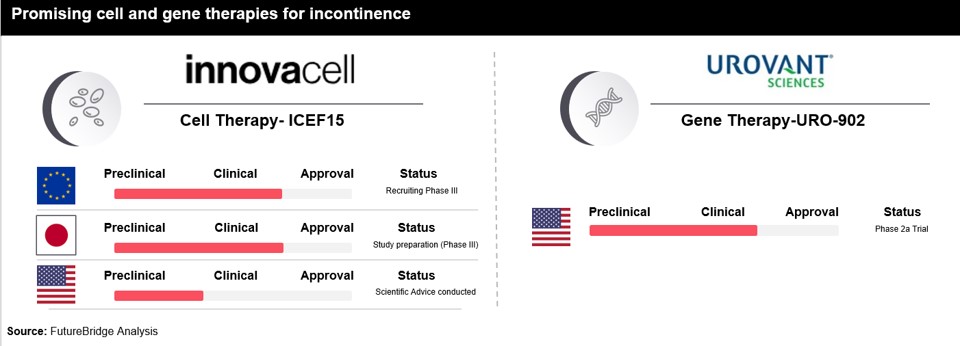
At the precipice of a medical revolution, cell and gene therapies are poised to redefine disease treatment, marking an $11 million market with a projected CAGR of ~25%. This nascent yet promising sector has the potential to revolutionize patient lives and possibly cure diseases. Projections indicate approval of over 10 novel cell and gene therapy products across the US and Europe in 2023, transcending beyond cancer and cardiovascular treatments to encompass incontinence. This therapy revolution is poised to change the medical landscape, innovating for better continence care, and impacting the future of continence care, leveraging the far-reaching impact of incontinence care innovation.
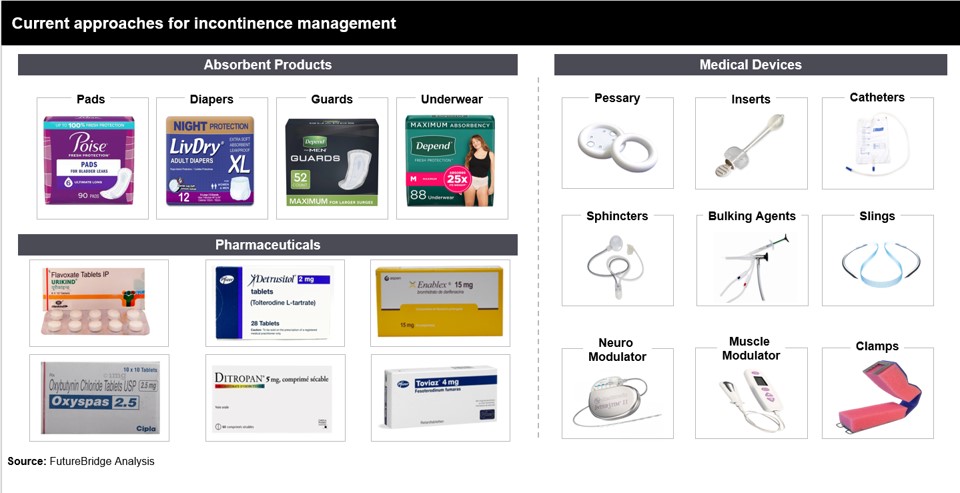
Current Approaches for Incontinence Management
Currently, the most used products are diapers and pads but cause discomfort to the patient as they become bulky while in use. Other conservative treatments, like Kegel exercises and lifestyle changes, were also the first line of. Overall, the segment lacked eco-friendly and tech-savvy options, paving the way for the current advancements in this sector. Today, incontinence management is more advanced with interventions like minimally invasive surgeries, nerve stimulation, discreet high-absorbency products, emerging sustainable, bio-degradable solutions, wearables, apps, and telemedicine. These empower patients to manage it more effectively, remotely, and in real-time. Notably, one of the major advancements in managing incontinence is the use of cell and gene therapy. While still in an immature phase, the enormous potential for future growth can be seen from ongoing research in innovative molecular techniques such as conventional and ex-vivo gene therapy.