New technologies & partnerships have opened up many dimensions to shorten the R&D path for future vaccines
Vaccine development has traditionally been a process measured in years and even decades as scientists experiment with a pathogen trying to weaken or dissimulate it to render it capable of creating an effective immune response with tolerable levels of side effects. All vaccines have the same basic goal: to train the body’s immune system to recognize and respond to a specific threat, whether chickenpox, polio, or SARS-CoV-2 (the virus responsible for COVID-19).
To accomplish this, most vaccines introduce a weakened or inactivated version of a virus (or some “recognizable” protein from a virus) so the body can learn to detect and defeat that virus. Pfizer and Moderna made headlines in 2020 for using a groundbreaking new approach that was years in the making. Rather than introducing a weakened copy of the coronavirus (or one of its recognizable proteins), their COVID-19 vaccines introduce strands of messenger RNA (mRNA).
mRNA — the basis of the first two vaccines cleared for public use by the Food and Drug Administration (FDA) — induces cells to set off an immune response against the coronavirus. The mRNA strands act like sets of instructions that tell the body’s cells to produce copies of a recognizable (but harmless) virus protein. In COVID-19’s case, SARS-CoV-2’s infamous “spike” protein. Vaccine scientists believe that the success of mRNA vaccines will bring an exponential change in the vaccine development lifecycle.
Change in the lifecycle of vaccine development
If we look at the new drugs developed since 2000, the trend shows that the mean development timeline from the start of clinical testing (Phase 1) to approval—is nearly ten years. This holds true even for any new anti-infective vaccines. For instance, the development timeline of vaccines for the human papillomavirus, shingles, and pneumococcal infections was between nine and 13 years. Most academics and pharmaceutical industry opinion leaders consider a development timeline of fewer than five years to be highly unusual.
It is, therefore, highly encouraging then, that three COVID-19 vaccines were granted emergency-use authorization (EUA) or other forms of approval in Europe, the United Kingdom, and the United States by the end of 2020. It was just 11 months after the COVID 19 sequence was first published and just eight or nine months after the first human doses were administered (Exhibit 1). The previous record for the fastest vaccine development was that for the mumps virus, which took four years (from sampling to approval) during the 1960s.
The vaccine scientists were exceedingly fortunate with COVID 19 in many aspects. Firstly unlike many other viruses COVID 19 virus does not mutate a lot and secondly, it doesn’t have effective strategies for foiling the human immune system, unlike HIV, herpes, or even influenza. This is as opposed to the herpes virus which by contrast, has more evasion capability as it actively blocks antibodies from binding, which makes it harder to find an effective agent against it. And the fast mutation of flu viruses requires a different vaccine formulation for every flu season.
The impetus didn’t directly start from the urgency of the COVID 19 pandemic itself. Previous infections such as Ebola, Zika, and MERS lead to the creation of international infrastructure that promotes information transfer and resource availability leading to global coordination and faster response time.
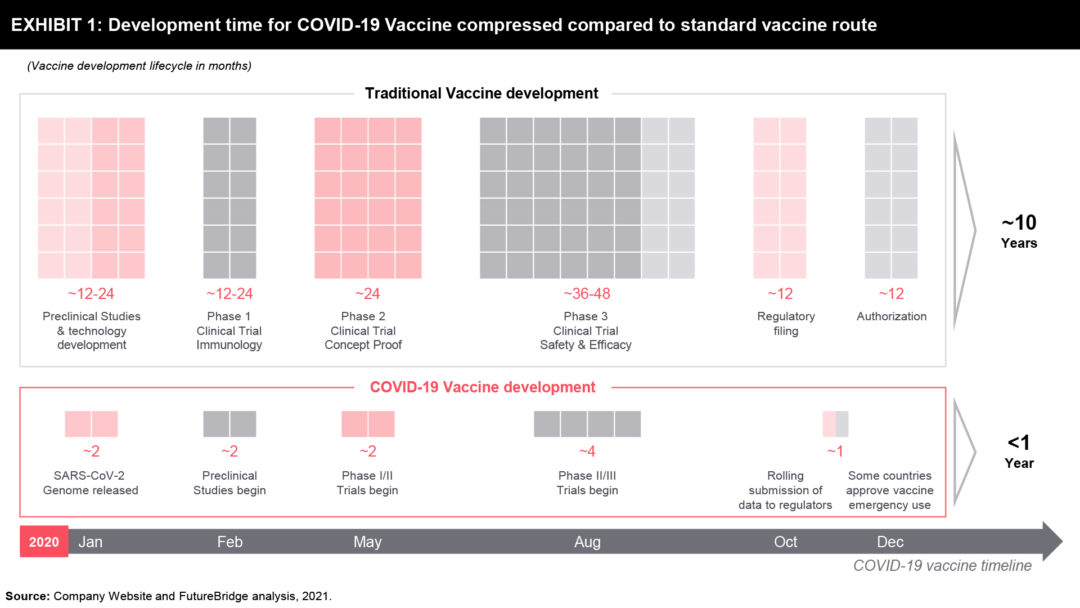

Years of Vaccine Research Advancements
The research that helped to develop vaccines against the new coronavirus didn’t start in January. For years, researchers had been paying attention to related coronaviruses, which cause SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome), and some had been working on new kinds of vaccine — an effort that has now paid off spectacularly.
The basic research on DNA vaccines began at least 25 years ago, and RNA vaccines have benefited from 10–15 years of strong research, as some vaccine development companies are targeting cancer vaccines. This approach of using RNA and DNA has matured just at the right time. Five years ago, the RNA technology would not have been ready and thus the development of any vaccine would have taken considerably more time.
The boost to COVID 19 vaccine development was possible for instance, because the researchers at the US National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland, knew from their research on MERS and SARS that it was best to tune the RNA sequence to stabilize the resulting spike protein in the form it adopts before it docks with a host cell.
The third vaccine to show efficacy in phase III clinical trials in November, made by the pharmaceutical firm AstraZeneca in collaboration with the University of Oxford, UK, does not use mRNA. Instead, a viral vector (or carrier) holds extra genetic material that codes for the SARS-CoV-2 spike protein. This, too, benefited from years of research in vector selection; in this case, the firm chose a modified form of adenovirus isolated from chimpanzee stool. Advances in conventional vaccines such as these have also come from research on SARS, MERS, Ebola, and malaria, and such approaches remain cheaper than using mRNA.
Ample funding
COVID 19 due to its global impact, made for the right situation for global collaboration. Usually, the slowest part of vaccine development isn’t finding candidate treatments, but testing them. This often takes years, with companies running efficacy and safety tests on animals and then on humans. Human testing requires three phases that involve increasing numbers of people and proportionately escalating costs. The COVID-19 vaccines went through the same trials, but the billions poured into the process made it possible for companies to take financial risks by running some tests at the same time.
With large sums given to vaccine firms by public funders and private philanthropists (Exhibit 2), they could conduct preclinical and phase I, II, and III trials, as well as manufacturing, in parallel instead of sequentially. This meant that companies could gamble on starting large-scale testing and manufacturing of candidates that might not work out.
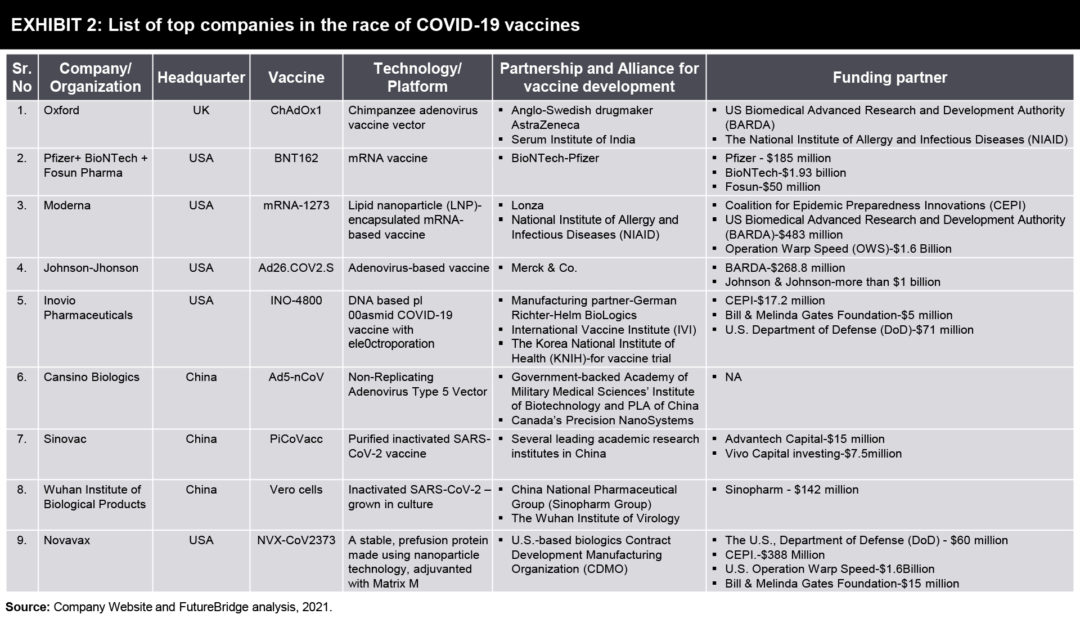

Non Traditional Vaccine Development for COVID 19
Today’s COVID-19 climate is both a challenging and enlightening time for the clinical development industry. The COVID vaccine race has advanced the acceptance of new technology and the international corporation has lead to a reduced burden on both sites and subjects. With real-time data access, faster decision-making and regulatory urgency have to lead to new technologies being available to the public rapidly as well as in a safe manner. The new technologies which have led to the rapid development of COVID vaccines and which will have a lasting effect on the industry are as follows.
Vaccines based on SARS-CoV-2 proteins
Viral surface proteins have been successfully used for designing various human vaccines (Exhibit 3). Unlike traditional methods of purification of these proteins from microbes, currently, they are manufactured in vitro with the help of recombinant DNA technology.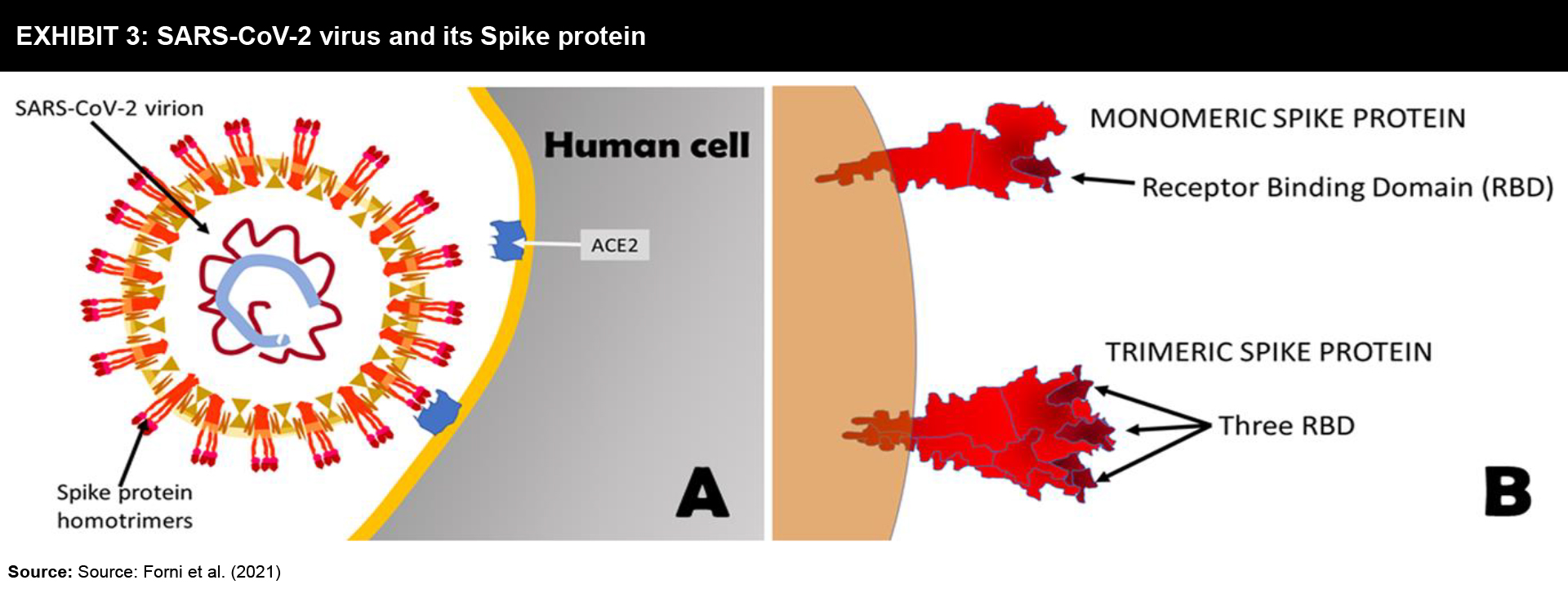
Strategy: The Spike protein that protrudes outside the SARS-CoV-2 virus is a trimeric aggregate that binds to human cells. Hence, many vaccines focus on Spike protein or its fragments as the main targets (Exhibit 4). These vaccines are used along with adjuvants in order to achieve a strong immune response.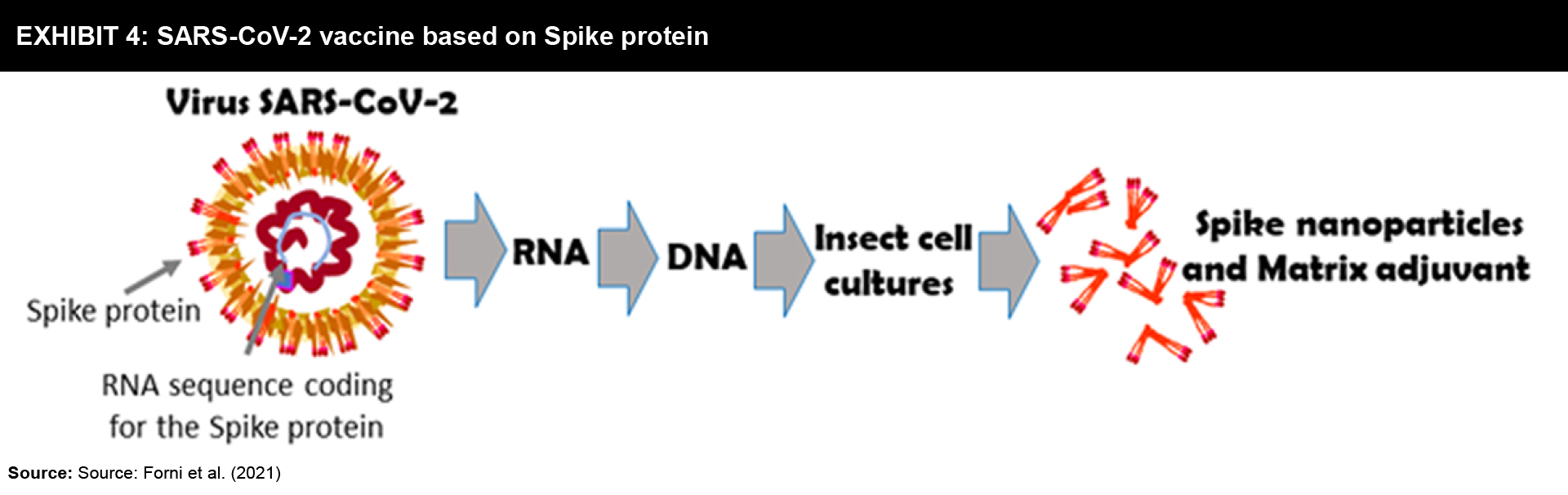
Prime developers: There are numerous vaccine projects based on SARS-CoV-2 virus proteins, their fragments, or combinations. At present, a minimum of sixteen candidate vaccines is under human trials and two in Phase II trials.
- Spike protein or its fragments plus adjuvant;
- Medien, Taiwan-US, plus Dynavax’s CpG adjuvant;
- Sanofi plus GlaxoSmithKline adjuvant, France – Italy;
- The University of Queensland, Australia;
- University of Tübingen, Germany;
- Biotechnology Vector, Russia;
- Clover Biopharmaceuticals plus GlaxoSmithKline adjuvant, China-Italy.
- Proteins carried by nanoparticles: Novavax, US, US, Australia, and South Africa, plus adjuvant.
DNA-based vaccines
The DNA and mRNA-based technologies provide ease of handling due to their stability and potential speed in large production in bacteria. However, till now DNA vaccines have not been registered for human use whereas they are frequently used in veterinary medicine.
Strategy: After intramuscular administration of these vaccines, DNA plasmids get into human cells, and making the cell synthesize the target protein provisionally. Plasmid entry can be boosted via electroporation. This induces the production of antibodies and subsequent triggering of killer T cells.
- Naked DNA plasmids:
- Zydus Cadila, India,
- AnGes, Japan,
- Takis, Italy.
- Naked DNA plasmids plus electroporation:
- Inovio, US,
- Genexine, Korea.
Vaccines based on mRNA technology
Although mRNA has not come up with any registered vaccine, there are quite a few vaccine developers utilizing this technique to design SARS-CoV-2 vaccines. In contrast to DNA, RNA must be delivered in many ways to move into the human cell. Then the mRNA vaccine temporarily prompts the cell to synthesize the mRNA-coded antigen protein (Exhibit 5).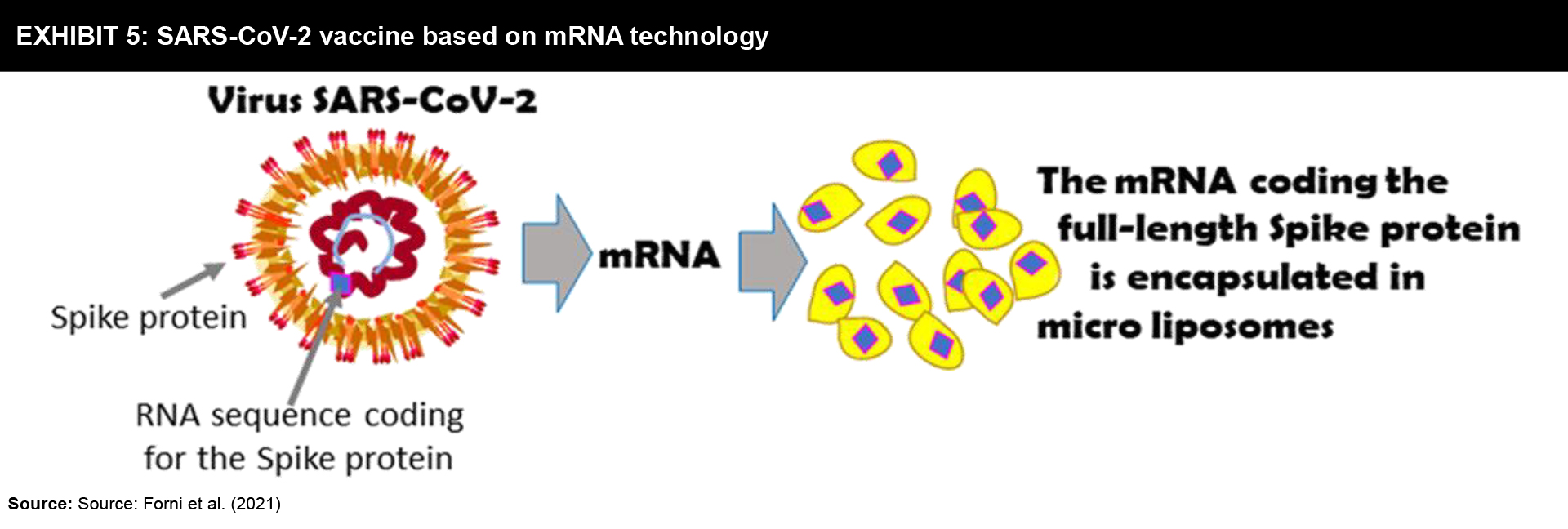
Strategy: Mostly, these vaccines are developed in which liposomes are used to deliver the mRNA. The anti-SARS-CoV-2 mRNA vaccines are designed to code for the Spike protein, its variants, or its fragments. The key concern about these vaccines is their storage at −30 to −80 °C which can be a constraint in transporting and distributing these vaccines to other countries.
Prime developers: Among many mRNA vaccines that code for Spike protein, its variants, or its fragments only two of those have completed Phase III trials.
- Lipid vesicles (Liposomes):
- Moderna, US,
- University of Oxford, UK,
- CureVac, Germany,
- Pfizer, US – BioNTech,
- Abogn, China.
- Nanoparticles: Arcturus Ther, Singapore.
Vaccines based on viral vectors
This platform exploits the ability of viral vectors to infect and deliver the DNA or mRNA into human cells. Ideally, adenovirus from chimpanzees or gorilla is used for this purpose as they become non-replicative after inserting the DNA (Exhibit 6). In this way, DNA coding for the SARS-CoV-2 Spike protein can be transported into the cells.
Strategy: Commonly, these virus-based vaccines are injected intramuscularly. However, various studies are underway to administer the vaccine intranasal. This candidate vaccine could stimulate a mucosal immunity that can nullify the virus by preventing its capability to move into the human system.
Prime developers: These viral vectors based candidate vaccines are already under progressive clinical trials and four of them are in Phase III trials now or permitted for restricted practice.
- Engineered non-replicating virus vectors
- Chimpanzee adenovirus: AstraZeneca, University of Oxford, Sweden-UK-Italy
- Gorilla adenovirus: ReiThera, Italy.
- Human adenoviruses: Johnson & Jonhson- US, CanSino- China, Gamaleya Research Institute- Russia.
- Engineered replicating virus vectors
- Measles virus: Merck, US
- Influenza virus: University Hong Kong, Valavax-Abogn, China, Beijing Vandal Biol Pharm, China.
A few other vaccine technologies:
- Symvivo, Canada: Oral administration of engineered Bifidobacterium probiotic that delivers the DNA encoding the Spike protein is under Phase I human trial
- Immunomonitor, Canada: heat-inactivated plasma from donors with COVID-19 is being verified under Phase I/II human trial
- Aivita Biomedical, US: Exploiting the patient’s modified dendritic cells to express SARS-CoV-2 antigens is under Phase I/II human trial.
Conclusion
Since last year many experts have tried hard and succeeded in developing vaccines for COVID-19. Adding to that, several minor biotech companies and university research laboratories are also involved in generating new vaccines through different technologies. Probably vaccines from these projects may turn out to be important if the leading vaccines do not work well in definite groups of the population or if they become unaffordable with unequal distribution in lower-income countries. Thus, the geographical features of the pandemic also determine the success of the vaccination strategy. For instance, nasal and oral vaccines appear to be interesting substitutes as they can prompt strong protection on the mucosal surfaces and could prevent the virus infection and spread through respiratory droplets.
Thus, positive authorization of the earliest available vaccines is not the only solution to contain the COVID-19 pandemic. Hence, new studies that result in second and third-generation vaccines should be encouraged with more financial support because extermination of COVID-19 may take a long duration and it will not finish with the available first vaccine. The country that produces the vaccine will give priority to safeguard its citizens and the residents of its friendly nations and thus the vaccine may turn out to be an incorrect measure of supremacy. Hence, it is important to manufacture surplus first doses of the vaccine that could lead to a rational supply to poor countries within a short period. In any case, more than one vaccine will be necessary to guarantee the protection of diverse populations, worldwide access, and cope up with new viral variants.
References
- A guide to global covid-19 vaccine efforts | Council on Foreign Relations
- Top 9 Companies leading the race of covid-19 vaccine | Intellizence
- Coronavirus vaccine Pipeline | Biopharma dive
- Landscape of covid-19 vaccine candidates | WHO
- Covid-19 vaccine frontrunners | The Scientist
- Pfizer & BioNTech concludes phase III study of covid-19 vaccine | Pfizer News Release
- Investors Earnings | Moderna
- Vaccine wagers on coronavirus surface protein payoff | Science
- SARS-CoV-2 receptor protein structure | Cell
- SARS-Cov-2 cell entry | Cell
- Covid-19 vaccines: where we stand and challenges ahead | Nature
- Covid-19 vaccinations: Progress | DW
Need a thought partner?
Share your focus area or question to engage with our Analysts through the Business Objectives service.
Submit My Business ObjectiveOur Clients
Our long-standing clients include some of the worlds leading brands and forward-thinking corporations.
- © 2026 Cheers Interactive (India) Private Limited. All rights reserved. FutureBridge ® is a registered trademark of Cheers Interactive (India) Private Limited.




































