Introduction
Why is there a need for developing a better bioactive delivery mechanism?
Providing nutrition/treatment for patients are complex in terms of three aspects:
- Understanding the mechanism and identifying the target for bioactive action
- Identifying and developing the bioactive molecule
- Delivering the bioactive to the target
Ongoing research has been able to solve the first two aspects and is almost successful with the third aspect too. However, the major hurdle for therapy is that the human body itself poses a challenge for effective targeting. Bioactives are either fragile or too big for cells and have difficulty in accessing the barriers in-vivo. Research on exosomes is in progress for the past two decades. It has been learned that exosomes are a natural solution for providing bioactive delivery.
Bioactives can act as delivery carriers and are capable of transporting various types of bio-macromolecules, such as protein, RNA therapeutics, and other small molecule drugs. Further, it is also possible for exosomes to be added with targeting molecules on the outside, which can help in identifying the target sites for action.
Exosomes are capable of accessing the difficult blood-brain barrier as they are small in size, but not too small to be expelled.
What are exosomes?
Exosomes are very small vesicles having an average size of 30-150nm. They are capable of carrying RNA and proteins in them. They are secreted by cells and perform various functions in the human body, such as intercellular communication and signaling. Exosomes are different from microvesicles in terms of formation, with microvesicles forming near the plasma membrane via membrane budding.
Exosomes comprise of membrane-bound intracellular proteins. The contents include membrane transport and fusion proteins, MHCs, heat shock proteins, tetraspanins, and others. Further, they comprise of proteins specific to the cell from which they are secreted.
How are exosomes formed?
Exosomes are formed from cells via a complex process and are then secreted by the cells. The elaborate production process is divided into three stages:
- Cell membrane invagination forms intracellular bodies
- Intracellular body polyvesicularization
- Multivesicular body and plasma membrane fusion to release exosomes
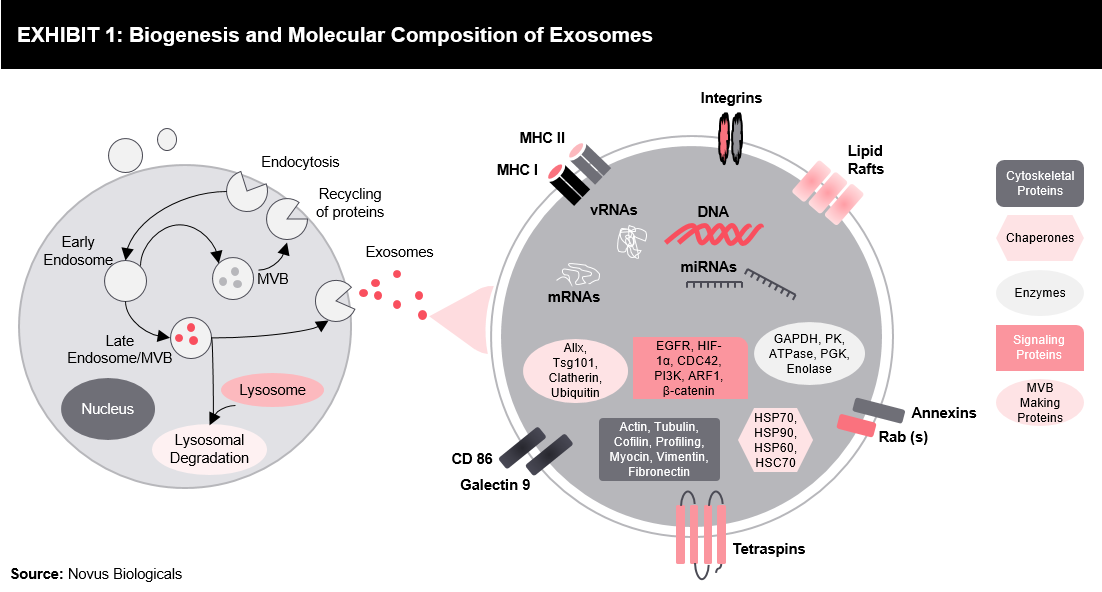
The process (refer Exhibit 1) starts with the formation of early endosomes by eukaryotic endocytosis. Endosomes are regulated by the Golgi apparatus to form the late endosomes. Invagination can be observed by the outer membrane of the late endosomes to form small vesicle structure, termed as Multivesicular Bodies (MVBs). Some of the MVBs are degraded by lysosome and some are transported to the Golgi apparatus for protein recovery. A few MVBs are fused with the plasma membrane to release small vesicles to extracellular. The released structures are termed as exosomes. The formation of exosomes is regulated by certain proteins, such as ESCRT, Rab, and other factors, such as amino acid concentration, pH, etc.
What are the functions of exosomes? What are the advantages?
Exosomes are biologically active vesicular structures containing liposomes, proteins, RNA, and miRNAs. They can be absorbed by surrounding cells and different tissue cells in the body fluid circulation.
They help in the transfer of substances and information between cells while participating in the regulation of gene expression levels of recipient cells and the physiological functions of recipient cells.
Exosomes are also involved in other biological functions, including immune regulation. The immune activity of exosomes can be observed as:
- Inducing T lymphocyte immune responses caused by exosome carrying antigen secreted by B lymphocytes
What makes exosomes efficient bioactive delivery tools?
The inherent properties of exosomes make them a suitable candidate for drug delivery. Exosomes avoid renal clearance due to their large size while being small enough to escape uptake by the reticuloendothelial system.
Exosomes accumulate at tumor sites due to the structure of the vasculature and abnormal lymphatic drainage. This specific characteristic is what makes them a suitable carrier for drug delivery in patients with cancer.
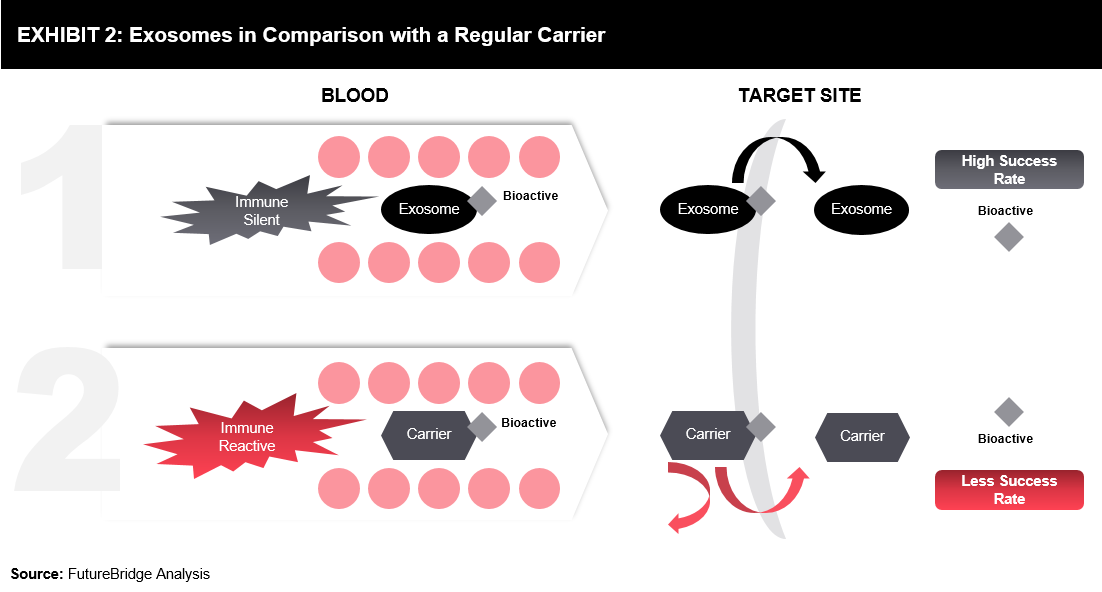
Two inherent characteristics of exosomes offer important opportunities for drug delivery (refer Exhibit 2)
- Immune Silent: Evades the immune system that allows exosomes to move through various sensors in the body, which is used to detect and halt the spread of foreign substances.
- Membrane composition: Complex membrane composition of exosomes allows them to deliver biological messages.
Exosomes are composed of two lipid bilayers:
- Forming an aqueous inner compartment, and
- Lipophilic outer layer
The membrane structure enables the loading of both hydrophobic and hydrophilic materials into exosomes. They can vary widely in the type and amount of cellular components they carry with certain lipids, proteins, and nucleic acids.
Exosomes contain high amounts of cholesterol, sphingolipids, phosphoglycerides, ceramides, and saturated fatty acid chains. The incorporation of these rigid molecules appears to contribute to the stability of exosomes.
Exosomes also contain nucleic acids, including microRNA, non-coding RNA, and messenger RNA. RNAs can be loaded into exosomes, either on the outside or inside, as they can be targeted and packed using a protein that can recognize the motifs in the RNA.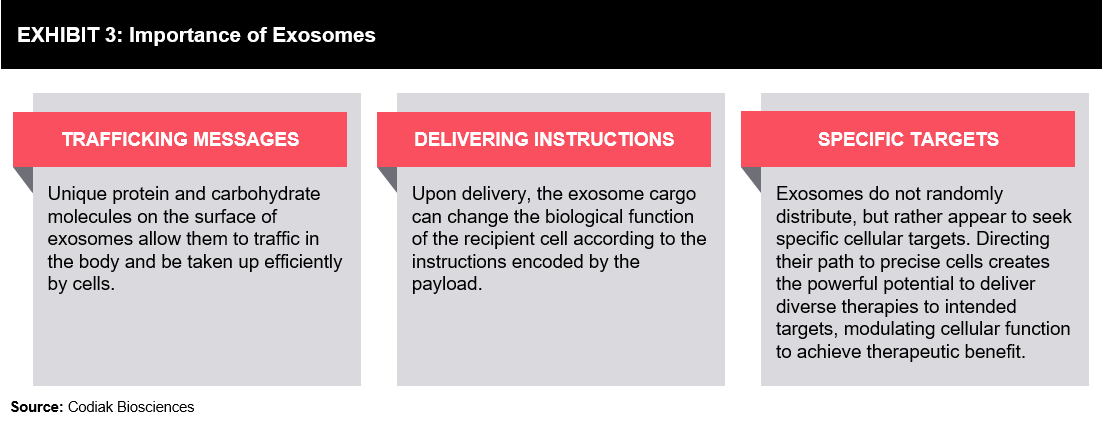
Active Entities
The exosome landscape has a mixed set of entities ranging from established players to start-ups. Some examples of established players in the landscape include Thermo Fisher Scientific (US), QIAGEN N.V. (Netherlands), Lonza (Switzerland), and Bio-Techne Corporation, along with other SMEs such as System Biosciences, LLC, and Diagenode.
Production of Exosomes
The start-up activity in the landscape is prominent. Examples of two UK-based startups who have developed the process for manufacturing exosomes are considered for explaining the production of exosomes.
The first example (refer Exhibit 4) looked at is of Codiak Biosciences. The start-up is based out of the UK and has developed the engEx Platform, which has been patented.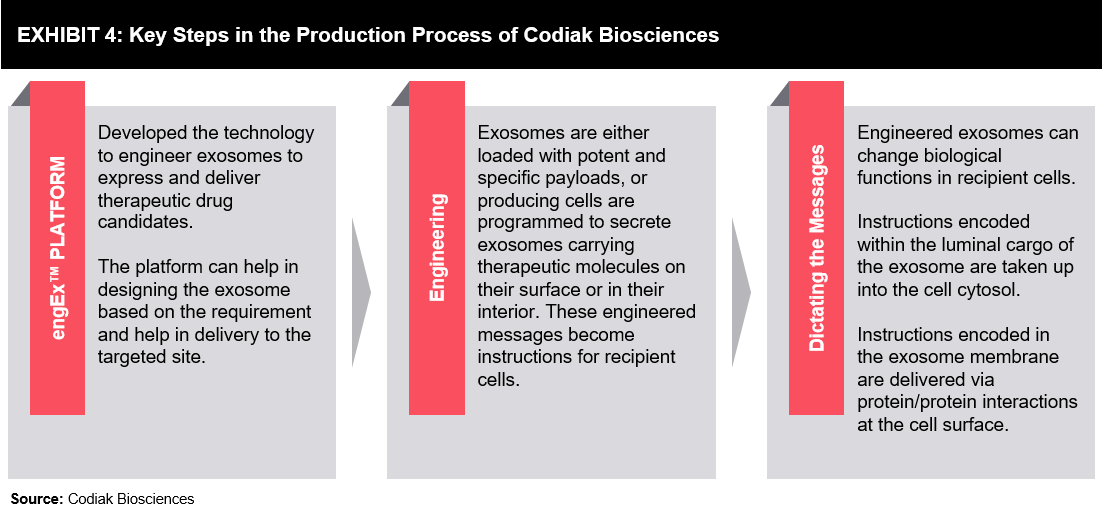
The process comprises of:
- Culturing a population of producer cells in a single-cell suspension
- Culturing in an animal-free serum
- Using tangential flow filtration perfusion or alternating tangential flow filtration perfusion to increase cell viability compared to cells cultured using a fed-batch culturing method cultured for the same number of days.
- Considering extracellular vesicle preparation comprises reduced proteinaceous contaminants, nucleic acid contaminants, small molecules, metabolites, and membranous contaminants.
The next example considered is of Evox Therapeutics Inc., a spin-out of Oxford University. The company has designed a large-scale production technology for purified exosomes.
The process comprises culturing of cells and key differentiators during the purification of exosomes, wherein the produced exosomes undergo ultrafiltration, followed by size-exclusion liquid chromatography.
For increasing the yield of the product, the culture is exposed to an autophagy inhibitor, wherein the process of destruction of exosomes is blocked to increase yield.
Applications of Exosomes
Current applications being researched
Exosomes are being majorly considered for their use in therapeutic applications. They are capable of stimulating immune responses and aiding in the development of vaccines to treat cancers.
Further, exosomes are potential biomarkers that can help in the detection of diseases and can be used for diagnostic purposes as they are found in body fluids.
One such application that is mainly being focused on is diagnostics. Exosomes provide a non-invasive mode of biopsy for professionals and reduce the risks and complications associated with biopsy.
Exosomes Diagnostics launched a disruptive technology in 2016 for detecting and gaining a real-time understanding of a cancerous tumor. The technology helps in understanding the tumor activity and enabling the detection of important changes. The technology was a major hit in the market, leading to its acquisition by Bio-Techne.
Possible avenues/applications
Exosomes are used for therapeutic and diagnostic purposes; however, the application of exosomes can be expanded to other areas due to their inherent properties and their ease of adaptation with human systems.
Research is being conducted on using exosomes via oral delivery to consumers. PureTech Health, a US-based company, is focusing on providing a delivery alternative using milk exosomes. The company specializes in the brain-immune-gut axis and is working on the oral administration of biologics, nucleic acids, and complex small molecules via exosomes. The advantage of milk-derived exosomes is that they can improve the oral bioavailability of macromolecules and complex small molecules.
Milk exosomes are evolutionarily-conserved microvesicles that are capable of passing through the stomach and into the GI tract due to their high integrity during transit.
Milk exosomes have a further advantage of being more durable in comparison to other exosomes. They have proved to be durable under acidic conditions.
In line with the development of milk exosomes, it can be predicted that milk exosomes can be possibly used in the nutrition segment. They can be availed for providing specialized nutrition, but the challenge lies in passing the approval process.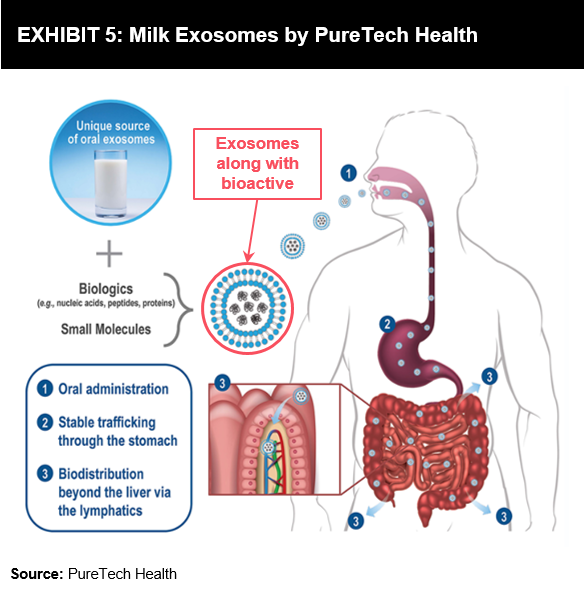
References
- https://www.novusbio.com/research-areas/cell-biology/Exosome-research-tools
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/exosomes.html
- https://www.creativebiomart.net/blog/overview-of-formation-and-function-of-exosomes-as-well-as-the-important-role-in-the-occurrence-and-deterioration-of-cancer/
- https://www.frontiersin.org/research-topics/4844/exosomes-role-in-cell-function-neurodegeneration-and-therapy
- https://www.evoxtherapeutics.com/Exosome-Technology
- http://www.codiakbio.com/science/
- https://www.mybeckman.in/resources/research-areas/exosomes/drug-delivery-with-exosome-nanoparticles
- https://www.britannica.com/science/exosome
- US20190085284A1
- US20160137716A1
Need a thought partner?
Share your focus area or question to engage with our Analysts through the Business Objectives service.
Submit My Business ObjectiveOur Clients
Our long-standing clients include some of the worlds leading brands and forward-thinking corporations.
- © 2021 Cheers Interactive (India) Private Limited. All rights reserved. FutureBridge ® is a registered trademark of Cheers Interactive (India) Private Limited.




































