Introduction to Corrosion and its Impact
Corrosion is a naturally occurring phenomenon where a metal deteriorates due to chemical or electrochemical reaction with its environment. The science of corrosion prevention and control is highly complex, worsened by the fact that corrosion takes several different forms and is affected by numerous external factors. These factors include the effects of environmental conditions, such as soil resistivity, humidity, and exposure to saltwater on various types of materials; the type of product to be processed, handled, or transported; required lifetime of the structure or component; proximity to corrosion-causing phenomena, such as stray current from rail systems; appropriate mitigation methods; and other considerations before determining the specific corrosion problem and specifying an effective solution.
Corrosion management is a key concern for all oil & gas assets. This is mainly due to the nature of fluids produced and injected throughout the assets lifecycle, regardless of the age of asset types and the level of corrosive agents present in the flow stream, be it CO2, H2S, water, or chloride. Extending the life of aging assets that are often degraded by corrosion is a major challenge that every operator is currently facing. Meanwhile, new greenfield development tapping hydrocarbons are confronted by highly corrosive contaminants, as the low-lying, easy wells have been mostly exploited.
Installed and running over several kilometers, pipelines carrying oil & gas are subjected to extreme weather, dirt, dust, and rain. Without any proper coating and absence of periodic maintenance, the chances of critical failure of these pipelines increase due to mass built-up and internal scaling.
The effect of corrosion in the oil industry leads to the failure of parts. This failure results in ceasing plant operations to clean the facility. According to NACE, the annual cost of corrosion to the oil & gas industry in the US is estimated to be $27 billion, whereas the global annual cost to the oil & gas industry is exceeding $60 billion.
Types of Corrosion
There are different types of corrosion that can occur on steel and metal components.
Basic Corrosion: Basic corrosion is a partially chemical and partially electrical process. A combination of moisture and oxygen is required for this process to begin. Once these two elements come together or are in contact with steel, a multi-stage process begins:
- Firstly, iron (Fe) atoms become positively charged by losing some electrons, and thus, have a high affinity for negatively-charged ions.
- Secondly, water (H2O) and oxygen (O) mix together and become even more negatively charged, thus attracting themselves to the positively-charged iron atoms. The result is a chemical known as iron hydroxide (4Fe (OH) 2).
- Lastly, iron hydroxide continues to react with oxygen, yielding 2Fe2O3.H2O, also known as hydrated iron oxide or brown rust.
As long as there is no barrier between the iron and water/oxygen molecules to stop this reaction, it continues reacting and converting iron into rust. The reasons are usually poor design, inappropriate material selection, and/or neglect in timely maintenance.
Bimetallic Corrosion: Bimetallic corrosion is the second type of corrosion that affects steel. This type of erosion occurs when a chemical reaction is caused by two metals coming in contact with one another. More common in metal alloys, this type of corrosion is quite complex, as there are multiple variations. The corrosion process is partly dependent on any two metals’ respective positions in the galvanic series. It happens most frequently in steel structures, which are buried or submerged.
Environmental Corrosion: Certain environmental pollutants, toxins, and compounds can aggravate either basic corrosion or bimetallic corrosion. This is the main reason why the location of the asset plays a vital role in material selection as well as protective coating selection. Thus, typical causes of corrosion on structural steel members include:
- Uncoated steel
- Steel that has not been coated with respect to the particular environment
- Steel that has not been adequately maintained
- Lack of a vapor barrier and/or adequate insulation inside the facility
- Unaddressed maintenance issues, such as plumbing leaks, standing water, etc., which lead to chronic exposure to moisture
Key Technologies and Solutions
Some of the leading coating types are mentioned below (refer Exhibit 1):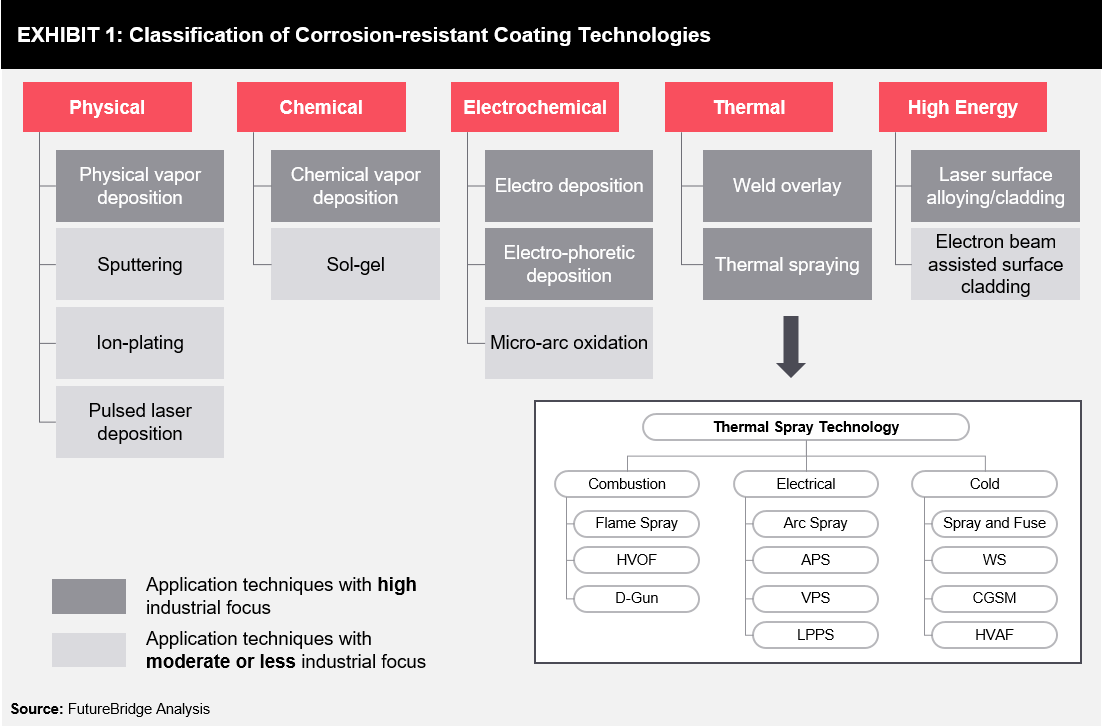
Physical Vapor Deposition (PVD): PVD is one of the versatile surface modification methods commonly used in the manufacturing of coatings, powders, fibers, and monolithic components. It is an atomistic deposition process where the target material is changed from a solid or liquid source (in the form of atoms or molecules) to form vapor in a vacuum or low-pressure gaseous (or plasma) environment, before it is changed back into a solid form and transferred to the substrate. While this technique provides a wide range of coating thickness, it can be used only in low-processing temperatures.
Chemical Vapor Deposition (CVD): CVD coatings are thin (sub-micron) and dense films that serve as a highly-effective barrier between the surface and corrosive media. These films are hydrophobic, anti-fouling, and chemically inert, as well as corrosion-resistant. They are used in several parts such as valves, heat exchanger coils, pumps, reactors, fittings and fasteners, gas transfer/delivery systems, downhole components, nozzles, tubing, and process sampling equipment.
Electrodeposition: In the electrochemical deposition process, a thin, metallic, inorganic, or organic coating can be electrochemically deposited on the surface of the substrate, protecting the substrate from the corrosive or erosive atmosphere. This process is relatively easy and less expensive when compared with other corrosion-resistant coatings options. A wide range of metals can be electrodeposited, thereby providing a broad range of functional coatings.
Thermal Coatings: Thermal coatings include weld overlay and thermal spray methods. In weld overlay, the substrate is protected by welding a compatible, high-performance material. In thermal spray, the melted material is sprayed onto the surface to provide a thick and robust coating.
Key Players in Oil & Gas Corrosion Protection
Solutions provided by some of the leading players (refer Exhibit 2) in this domain include:
Sherwin Williams: (mainly active in chemical-based solutions)
- Offers ControlTech linings for tanks, clarifiers, drains, and floors, which contain low VOC
- Heat resistant coatings: Offers corrosion-resistant products that resist cyclical wet/dry high temperature and corrosion environments for piping requiring Coatings Under Insulation (CUI)
- Optically activated pigments: This technology allows tank lining applicators to identify pinholes, thin spots instantly, or any other discontinuities using safe UV light
Hempel: The Company provides solutions for hot-dip galvanized steel, primed steelwork, and stainless steel. It has developed solutions specifically for upstream onshore and offshore as well as downstream businesses.
Jotun: The Company provides paints and coating solutions for dry storage, jetties, liquid and gas storage, pipelines, pressure vessels, and structural steel.
RPM: The Company provides solvent-free epoxy tank linings for handling crude, process liquids, and solvents for the midstream, whereas epoxy and polyurethane-based coatings and finishes for the upstream environment.
Key Research Directions
- Inventors are focusing on reducing the impact of CO2 on the surface of steel structures and pipelines. Dissolved CO2 bounds to react with low carbon steel in spite of coating with corrosion inhibitor; the reaction process can be controlled with the addition of chromium. One such Cr-containing low carbon steel, typically containing 2–2.5 wt% Cr, is used widely for forged components in a quenched and tempered heat-treated state.
- Al2O3 particles, in combination with epoxy coatings, are being tested to protect subsea umbilical cables. With micro-Al2O3 particles on the polymeric matrix, corrosion and wear properties are expected to be improved without a significant impact on the cost as compared with unmodified epoxy coating. This coating is believed to act as a barrier to electrolyte penetration without affecting the tribological properties.
- Digital technologies are deployed for predicting and preventing corrosion. These technologies can monitor crucial parameters, such as pH level and chlorides, along with corrosion probes. This helps in sending an early warning signal for chemical injections to control or mitigate the corrosion process. Robotic systems, UAVs, and high-definition cameras are used to identify and repair defects in a timely manner.




































